
Vial Inspection Machine: Ensuring Pharmaceutical Product Integrity
In the highly regulated pharmaceutical industry, ensuring the safety, quality, and efficacy of injectable drugs is paramount. Any particulate matter, cosmetic defect, or fill-level inaccuracy in a parenteral vial can pose serious health risks to patients. This is where vial inspection machines play a critical role. These sophisticated automated systems are designed to detect and reject defective products with unparalleled precision and speed, serving as an essential final checkpoint before medicines reach consumers.

Core Technology: How Automated Vial Inspection Works
Modern vial inspection machines utilize a combination of advanced technologies to perform 100% inspection of every vial produced. The process typically involves several stages of inspection, each targeting specific types of defects.
Machine Vision Systems
At the heart of any vial inspection machine is a high-resolution machine vision system. This consists of one or more high-speed cameras equipped with specialized lenses and lighting configurations. Strobe lights, often LED-based, freeze the motion of vials as they rotate on a turntable or conveyor, allowing cameras to capture multiple images from different angles. Advanced image processing algorithms then analyze these images in real-time to identify anomalies.

Defect Detection Algorithms
The software powering these machines is incredibly sophisticated. Using techniques like neural networks and deep learning, the algorithms are trained to distinguish between acceptable variations and critical defects. They can detect a wide range of issues, including:
- Particulate matter (glass, fibers, rubber, etc.)
- Cracks, chips, and other container defects
- Fill level inaccuracies
- Cloudiness or foreign solutions
- Cap placement and seal integrity issues
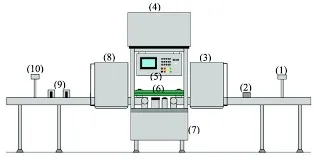
Key Components of a Vial Inspection Machine
A typical automated inspection system comprises several integrated components that work in unison to ensure comprehensive inspection.
| Component | Function | Importance |
|---|---|---|
| Infeed/Outfeed Mechanism | Controls the flow of vials into and out of the inspection station | Ensures proper handling and prevents bottlenecks in production |
| Rotation System | Spins each vial during inspection | Allows cameras to capture the entire inner surface for particulate detection |
| Vision System (Cameras & Lighting) | Captures high-quality images of each vial | The primary sensing system for defect detection |
| Rejection Mechanism | Removes defective vials from the production line | Prevents contaminated or defective products from proceeding to packaging |
| Human-Machine Interface (HMI) | Allows operators to configure and monitor the inspection process | Provides control and access to inspection data and results |
| Computing Hardware & Software | Processes images and makes accept/reject decisions | The "brain" of the system that executes complex detection algorithms |
Inspection Criteria and Regulatory Compliance
Vial inspection machines are designed to meet stringent regulatory requirements set forth by agencies like the FDA (U.S. Food and Drug Administration) and EMA (European Medicines Agency). These regulations are outlined in documents such as USP <1> and USP <790>, which specify visible particulate matter standards in injections.
Critical Quality Attributes Inspected
The inspection parameters are comprehensive and cover multiple quality attributes:
| Inspection Type | Defects Detected | Technology Typically Used |
|---|---|---|
| Top/Bottom Inspection | Cracks, mold marks, uneven finishes | High-resolution cameras with top and bottom lighting |
| Sidewall Inspection | Scratches, stains, engraving defects | Multiple cameras capturing circumferential views |
| Particulate Inspection | Foreign particles, glass fragments, fibers | High-speed cameras with backlighting |
| Fill Level Inspection | Overfills, underfills | Laser-based sensors or vision systems |
| Cap/Closure Inspection | Misplaced caps, missing flip-offs, seal integrity | Color cameras with specific angle lighting |
Benefits of Automated Vial Inspection
The adoption of automated inspection systems offers significant advantages over traditional manual inspection methods.
Enhanced Detection Accuracy and Consistency
Machines do not suffer from fatigue, distraction, or the subjectivity that affects human inspectors. They apply the same precise criteria to every single vial, 24/7, ensuring consistent and reliable inspection results.
Increased Production Throughput
Modern AVI systems can inspect thousands of vials per hour, far exceeding the capacity of human teams. This high-speed operation helps pharmaceutical companies meet production targets without compromising on quality control.
Comprehensive Data Collection and Traceability
These systems generate detailed reports and statistical process control (SPC) data, providing valuable insights into production quality trends. This data is crucial for continuous improvement efforts and for demonstrating compliance during regulatory audits.

Future Trends in Vial Inspection Technology
The field of automated inspection continues to evolve, with several emerging trends shaping the next generation of equipment.
Artificial Intelligence and Deep Learning
AI is revolutionizing defect detection by enabling systems to learn from vast datasets of images, continuously improving their accuracy and ability to identify subtle or complex defects that were previously challenging to detect.
Hybrid Inspection Models
Some systems now combine multiple inspection technologies, such as combining visual inspection with spectroscopic analysis, to detect not just visible defects but also chemical contaminants or material inconsistencies.
Integration with Industry 4.0
Modern vial inspection machines are becoming nodes in smart factory networks, exchanging data with other equipment (like filling machines and lyophilizers) to enable predictive maintenance, optimize overall equipment effectiveness (OEE), and create a fully integrated, data-driven manufacturing environment.
In conclusion, vial inspection machines are indispensable assets in pharmaceutical manufacturing, providing the critical final assurance of product quality and patient safety. As technology advances, these systems will become even more intelligent, integrated, and essential to the production of life-saving injectable medicines.
